Sunday, November 15, 2015
Sg Chem 2A!!!
This week in chem was particularly hard for me. We learnt lots of new material in a short amount of time.
Tuesday, November 3, 2015
Sg chem 2a
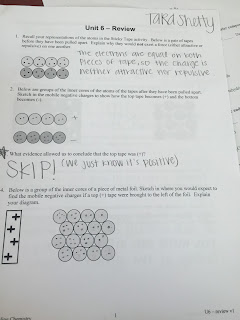
We didn't do much this week in chem. We started off by learning about Polyatomic Ions. Polyatomic atoms are groups of atoms that carry a charge (and are found regularly in food, natural waterways, etc). In the worksheet we did, we had to identify and place certain elements into different tables. At first I was really confused. I didn't even really understand what an Ion was, but after we white boarded and discussed it as a class, things started to make sense. After that, we did Unit 6 Worksheet 1. In this worksheet we learnt about positively charged ions (cations) and negatively charged ions (anions) and we had to the write the formula and draw the particle diagram for each compound. This was pretty straightforward and as a table we were able to fly right through it! However, there was one difficult part. It was on the second page and you had to write each formula (from the previous page) in the boxes corresponding to its elements. This was really weird and hard for a lot of us to understand, but after Dr. J went over it as a class, it started to clear up. From this table we were then able to predict the formulas of the compounds formed by the ions below (for example: __potassium
___oxygen etc). After we got the basics down we started doing a bunch more worksheets. There was one (worksheet 3, if we are being specific) where we had to give the name of the following binary ionic compounds, write the formula for the binary compounds, write the formula for ionic substances, write the name using roman numerals, and more. Each table was assigned specific questions and then we white-boarded them and went over all of them as a class. The material itself is not too difficult, but going over them as a class definitely helped. It was also comforting that a lot of people were making the same mistakes as you. The next worksheet we did was about representing ions and formula units. The front side was extremely easy and straightforward, but the back was a little more complicated. On the back we had to also state the number of atoms and the number of ions in the compound, and honestly, I'm not completely sure how to do that. We worked on it as a table, but I wish we could've done it as a class. After this worksheet, we started to review for our upcoming test. We got a blue sheet with all the symbols and charges for Polyatomic Ions and that was a LIFE SAVER!!! That made pretty much everything really simple, because then we didn't have to try and memorize everything. For the unit 6 review, we were all assigned a problem and then we white-boarded like before. There were a couple things we skipped, but we basically went over everything. I think the hardest questions that I didn't understand were the last couple (for example: why do chemists refer to CO2 as carbon dioxide, yet use the name tin(IV) oxide to describe SnO2?), but at the end it told us exactly what to study and I think that was nice. It reminded us to be familiar with the names, formulas and charge of the common ions, know which combinations of elements give rise to ionic compounds and more. We also were given some extra practice that helped a lot too. Overall, I think this was a good week and I cannot wait to keep learning!! :)))
___oxygen etc). After we got the basics down we started doing a bunch more worksheets. There was one (worksheet 3, if we are being specific) where we had to give the name of the following binary ionic compounds, write the formula for the binary compounds, write the formula for ionic substances, write the name using roman numerals, and more. Each table was assigned specific questions and then we white-boarded them and went over all of them as a class. The material itself is not too difficult, but going over them as a class definitely helped. It was also comforting that a lot of people were making the same mistakes as you. The next worksheet we did was about representing ions and formula units. The front side was extremely easy and straightforward, but the back was a little more complicated. On the back we had to also state the number of atoms and the number of ions in the compound, and honestly, I'm not completely sure how to do that. We worked on it as a table, but I wish we could've done it as a class. After this worksheet, we started to review for our upcoming test. We got a blue sheet with all the symbols and charges for Polyatomic Ions and that was a LIFE SAVER!!! That made pretty much everything really simple, because then we didn't have to try and memorize everything. For the unit 6 review, we were all assigned a problem and then we white-boarded like before. There were a couple things we skipped, but we basically went over everything. I think the hardest questions that I didn't understand were the last couple (for example: why do chemists refer to CO2 as carbon dioxide, yet use the name tin(IV) oxide to describe SnO2?), but at the end it told us exactly what to study and I think that was nice. It reminded us to be familiar with the names, formulas and charge of the common ions, know which combinations of elements give rise to ionic compounds and more. We also were given some extra practice that helped a lot too. Overall, I think this was a good week and I cannot wait to keep learning!! :)))
Subscribe to:
Posts (Atom)